Technology Transfer in the Pharmaceutical Industry: Driving Innovation
Technology transfer in pharmaceutical industry – Technology transfer in the pharmaceutical industry is the process of moving new discoveries and technologies from the research lab to the marketplace. This crucial […]
Technology transfer in pharmaceutical industry – Technology transfer in the pharmaceutical industry is the process of moving new discoveries and technologies from the research lab to the marketplace. This crucial step involves a complex interplay of stakeholders, including research institutions, pharmaceutical companies, and regulatory bodies. It’s the bridge between scientific breakthroughs and life-saving treatments, propelling the development of new drugs and therapies that address unmet medical needs.
The pharmaceutical industry thrives on innovation, and technology transfer plays a vital role in bringing new discoveries to patients. It involves the transfer of knowledge, expertise, and resources, often through licensing agreements, joint ventures, or spin-offs. Each approach presents unique advantages and challenges, requiring careful consideration and strategic planning for success.
Introduction to Technology Transfer in the Pharmaceutical Industry
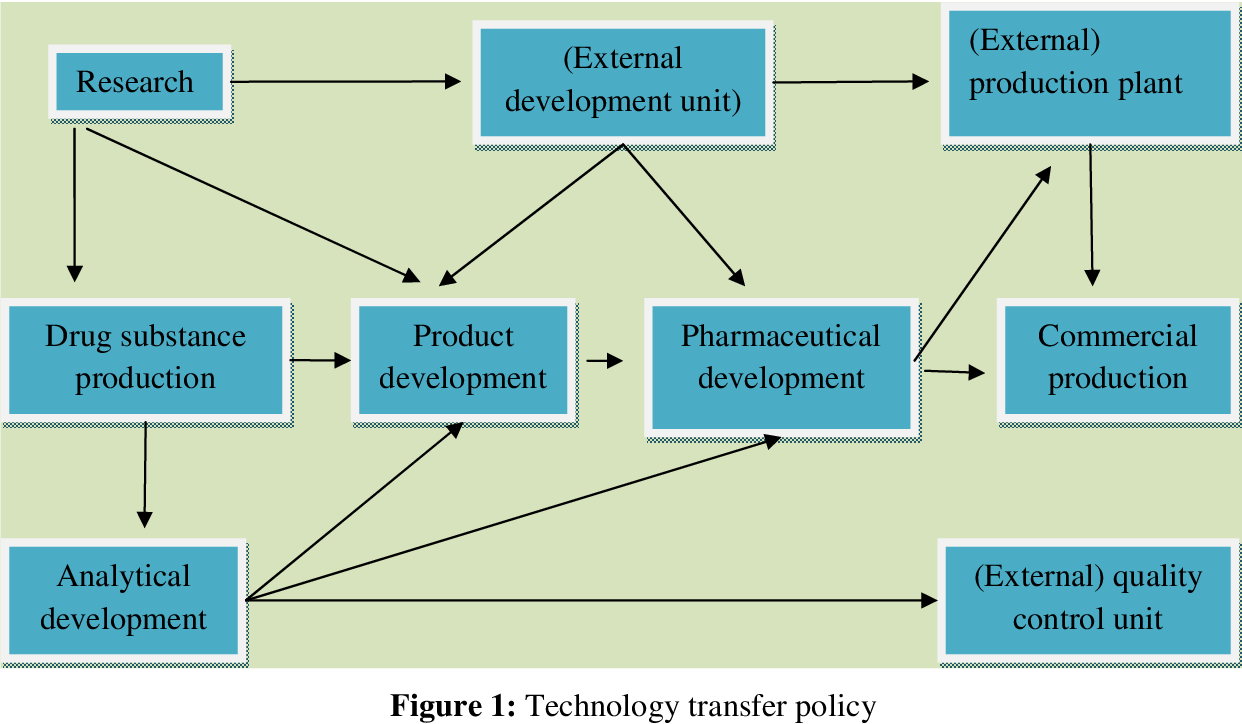
Technology transfer in the pharmaceutical industry is the process of moving a new technology, such as a drug candidate or a manufacturing process, from the research and development (R&D) stage to commercialization. This process involves transferring knowledge, skills, and expertise from one entity, typically a research institution or a pharmaceutical company, to another, which might be a different company, a manufacturing facility, or a regulatory body.
Technology transfer is a complex and multifaceted process that requires careful planning and execution. It involves a wide range of stakeholders, including scientists, engineers, regulatory experts, and business professionals. Effective technology transfer ensures that new technologies are developed, tested, manufactured, and marketed successfully.
Key Stakeholders in Technology Transfer
Technology transfer in the pharmaceutical industry involves several key stakeholders who play critical roles in ensuring the successful transition of new technologies from the research lab to the market. These stakeholders work collaboratively to navigate the complex regulatory landscape, optimize manufacturing processes, and ultimately bring life-saving therapies to patients.
- Research Institutions: These institutions are responsible for generating new knowledge and developing innovative technologies. They play a crucial role in the early stages of technology transfer by identifying promising candidates for further development.
- Pharmaceutical Companies: These companies are responsible for commercializing new technologies. They invest in research and development, conduct clinical trials, manufacture drugs, and market them to healthcare providers and patients.
- Regulatory Bodies: These bodies, such as the Food and Drug Administration (FDA) in the United States, are responsible for ensuring the safety and efficacy of new drugs and medical devices. They establish regulations and guidelines that pharmaceutical companies must adhere to during the technology transfer process.
- Contract Research Organizations (CROs): CROs provide specialized services to pharmaceutical companies, such as clinical trial management, data analysis, and regulatory support. They play a vital role in facilitating technology transfer by providing expertise and resources to support the development and commercialization of new technologies.
- Manufacturing Facilities: These facilities are responsible for producing drugs and medical devices according to strict quality standards. They play a critical role in technology transfer by ensuring that new technologies are manufactured consistently and reliably.
Importance of Technology Transfer for Innovation and Drug Development
Technology transfer is essential for innovation and drug development in the pharmaceutical industry. It allows for the efficient transfer of knowledge and expertise, which is crucial for bringing new therapies to patients quickly and safely.
- Accelerates Drug Development: Technology transfer streamlines the process of developing and commercializing new drugs by ensuring that the necessary knowledge and expertise are available at each stage of the process. This helps to reduce development timelines and bring new therapies to market faster.
- Enhances Collaboration and Knowledge Sharing: Technology transfer fosters collaboration between research institutions, pharmaceutical companies, and other stakeholders. This collaboration allows for the sharing of knowledge and expertise, which can lead to more efficient and effective drug development.
- Promotes Innovation: Technology transfer encourages innovation by providing a pathway for new technologies to be developed and commercialized. This can lead to the development of novel drugs and therapies that address unmet medical needs.
- Improves Patient Access to New Therapies: Technology transfer plays a crucial role in ensuring that patients have access to new and innovative therapies. By facilitating the development and commercialization of new drugs, technology transfer helps to improve patient outcomes and quality of life.
Types of Technology Transfer in Pharmaceuticals: Technology Transfer In Pharmaceutical Industry
Technology transfer in the pharmaceutical industry encompasses various methods for transferring knowledge, skills, and technologies from one entity to another. This transfer can occur between research institutions, pharmaceutical companies, or even different departments within the same organization. Understanding the different types of technology transfer is crucial for navigating the complex landscape of pharmaceutical innovation and commercialization.
Licensing Agreements
Licensing agreements are a common method for transferring intellectual property rights, such as patents, trademarks, and know-how, from one party (licensor) to another (licensee). The licensee obtains the right to use the licensed technology for a specific period and within a defined geographical area.
- Advantages: Licensing agreements allow companies to leverage existing technologies without incurring the high costs of research and development. This approach can be particularly beneficial for smaller companies seeking to access cutting-edge technologies or for companies expanding into new markets.
- Disadvantages: Licensing agreements can limit the licensee’s control over the technology and its commercialization. The licensor may also retain certain rights, such as the right to approve the licensee’s manufacturing processes or marketing strategies.
Joint Ventures
Joint ventures involve the creation of a new entity by two or more parties to jointly develop, manufacture, or commercialize a specific product or technology. This collaboration allows companies to pool resources, expertise, and market access.
- Advantages: Joint ventures can provide access to complementary expertise, shared resources, and a larger market reach. They also offer a higher level of control over the technology compared to licensing agreements.
- Disadvantages: Joint ventures require careful coordination and communication between the partners. Differences in business objectives, cultures, or decision-making processes can lead to challenges in managing the joint venture.
Spin-offs
Spin-offs occur when a new company is formed from an existing organization, typically based on a specific technology or product developed within the parent company. The spin-off company operates independently, focusing on the commercialization of the transferred technology.
- Advantages: Spin-offs provide a dedicated focus on the development and commercialization of a specific technology. They can attract investment and expertise specifically tailored to the new venture.
- Disadvantages: Spin-offs require significant investment and resources to establish themselves as independent entities. They may face challenges in securing funding, building a brand, and competing with established companies.
Research Collaborations
Research collaborations involve partnerships between two or more organizations to jointly conduct research and development activities. These collaborations can focus on specific projects, sharing research data, or developing new technologies.
- Advantages: Research collaborations provide access to diverse expertise, shared resources, and potential for faster innovation. They can also facilitate the exchange of knowledge and best practices.
- Disadvantages: Research collaborations require effective communication and coordination between the partners. Differences in research methodologies, intellectual property rights, or publication policies can create challenges in managing the collaboration.
Challenges of Technology Transfer in Pharmaceuticals
Technology transfer in the pharmaceutical industry, while crucial for innovation and product development, is not without its complexities and challenges. The intricate nature of pharmaceutical manufacturing, coupled with stringent regulatory requirements and intellectual property concerns, makes technology transfer a demanding process.
Intellectual Property Protection
Protecting intellectual property is paramount in the pharmaceutical industry, where innovation drives profitability and competitive advantage. During technology transfer, the transferor must carefully balance the need to share knowledge with the need to safeguard their proprietary technology.
- Licensing Agreements: These agreements define the terms of technology transfer, including the scope of intellectual property rights granted to the recipient. They specify the duration of the agreement, royalties, and obligations of both parties.
- Confidentiality Agreements: These agreements are essential for protecting sensitive information, such as manufacturing processes, formulations, and data. They establish clear confidentiality obligations for both parties, preventing unauthorized disclosure of confidential information.
- Trade Secret Protection: Trade secrets, such as specific manufacturing techniques or formulations, can be protected without formal patent registration. However, maintaining the secrecy of trade secrets requires robust security measures and ongoing vigilance to prevent unauthorized disclosure.
For example, a pharmaceutical company transferring a novel drug manufacturing process to a contract manufacturing organization (CMO) might need to negotiate a comprehensive licensing agreement that clearly defines the scope of intellectual property rights granted to the CMO, including restrictions on using the technology for other products or sharing it with third parties.
Regulatory Compliance, Technology transfer in pharmaceutical industry
The pharmaceutical industry is subject to stringent regulatory oversight, ensuring the safety, efficacy, and quality of drugs. Technology transfer must comply with regulatory requirements in both the origin country and the recipient country.
- Good Manufacturing Practices (GMP): GMP regulations dictate the standards for manufacturing, quality control, and documentation. Both the transferor and recipient must adhere to GMP requirements throughout the technology transfer process.
- Regulatory Approvals: Transferring a manufacturing process to a new facility often requires regulatory approvals, such as changes to marketing authorizations or manufacturing licenses. This process can be time-consuming and complex, requiring thorough documentation and inspections.
- Quality Assurance and Control: Maintaining the quality of the drug product is crucial. Technology transfer must include robust quality assurance and control measures to ensure that the transferred process meets the required standards.
For instance, transferring a drug manufacturing process from the United States to India requires ensuring compliance with both the FDA (Food and Drug Administration) regulations in the US and the CDSCO (Central Drugs Standard Control Organisation) regulations in India. This may involve site inspections, audits, and documentation reviews to demonstrate compliance with both sets of regulations.
Technical Know-How Transfer
Effective technology transfer requires transferring not just the technical documentation but also the tacit knowledge and expertise accumulated over time.
- Training and Documentation: Providing comprehensive training programs and detailed documentation is crucial for the recipient to understand the transferred technology. This includes hands-on training, process simulations, and detailed SOPs (Standard Operating Procedures).
- Knowledge Sharing: Establishing effective communication channels and fostering knowledge sharing between the transferor and recipient is essential for ensuring a smooth transfer. This can involve regular meetings, technical support, and access to experts.
- Troubleshooting and Problem Solving: During the technology transfer process, technical challenges are inevitable. Having a robust troubleshooting mechanism and access to technical expertise from both the transferor and recipient is critical for resolving issues quickly and efficiently.
For example, transferring a complex fermentation process for a biopharmaceutical product might require extensive training for the recipient’s personnel on the intricacies of fermentation technology, including troubleshooting common issues, optimizing process parameters, and maintaining aseptic conditions.
Cultural Differences
Technology transfer often involves collaborating with partners from different cultural backgrounds, which can present challenges in communication, work styles, and decision-making processes.
- Communication Styles: Differences in communication styles, such as directness or indirectness, can lead to misunderstandings. Building a strong foundation of trust and understanding through clear communication is crucial.
- Work Ethic and Values: Differences in work ethic, values, and decision-making styles can affect the effectiveness of technology transfer. Fostering a collaborative and respectful work environment is important for overcoming these challenges.
- Cultural Sensitivity: Demonstrating cultural sensitivity and respect for the recipient’s culture is essential for building strong relationships and facilitating a successful transfer.
For instance, transferring a manufacturing process from a Western company to a Chinese company might require adapting communication styles to the Chinese cultural context, which emphasizes indirect communication and building strong personal relationships.
Communication Gaps
Clear and effective communication is crucial for a successful technology transfer. Communication gaps can arise due to language barriers, technical jargon, or differences in understanding.
- Language Barriers: Language barriers can hinder effective communication and understanding. Using interpreters, translators, and clear written documentation can help overcome these challenges.
- Technical Jargon: Using technical jargon unfamiliar to the recipient can create confusion and misunderstandings. Using plain language and providing clear definitions of technical terms can help ensure effective communication.
- Different Levels of Understanding: The transferor and recipient may have different levels of understanding of the technology. Ensuring clear and consistent communication across all levels of the team is crucial.
For example, transferring a complex analytical method for drug quality control might require using clear and concise language to explain the methodology to the recipient’s analysts, ensuring they understand the principles, procedures, and data interpretation.
Strategies for Successful Technology Transfer

Successful technology transfer in the pharmaceutical industry requires a strategic and structured approach. This involves implementing a framework that addresses key areas, including due diligence and risk assessment, clear documentation and communication protocols, training and capacity building, effective project management, and ongoing monitoring and evaluation.
Due Diligence and Risk Assessment
Before initiating technology transfer, a thorough due diligence and risk assessment process is crucial. This helps identify potential challenges and mitigate risks associated with the transfer.
- Comprehensive Evaluation: Evaluate the technology’s feasibility, commercial viability, and regulatory compliance. This includes assessing the intellectual property landscape, manufacturing capabilities, and potential market opportunities.
- Risk Identification: Identify potential risks associated with the transfer, such as technical challenges, regulatory hurdles, and intellectual property infringement. Examples of potential risks include inadequate training of personnel, lack of appropriate infrastructure, and insufficient quality control procedures.
- Mitigation Strategies: Develop and implement strategies to mitigate identified risks. This may involve securing necessary regulatory approvals, investing in infrastructure upgrades, or providing comprehensive training programs.
Clear Documentation and Communication Protocols
Clear and consistent documentation and communication protocols are essential for a smooth technology transfer process.
- Detailed Transfer Protocol: Develop a comprehensive transfer protocol that Artikels all aspects of the transfer, including timelines, responsibilities, and deliverables. This document should clearly define the scope of the transfer, including the specific technologies, processes, and equipment involved.
- Standard Operating Procedures (SOPs): Establish clear and standardized operating procedures for all aspects of the technology transfer process. This ensures consistency and reproducibility of results.
- Regular Communication: Maintain open and regular communication between all parties involved in the transfer. This includes regular meetings, progress reports, and feedback mechanisms. This allows for timely identification and resolution of any issues or concerns.
Training and Capacity Building
Training and capacity building are essential to ensure the successful adoption and implementation of the transferred technology.
- Targeted Training Programs: Develop and deliver targeted training programs for all personnel involved in the technology transfer process. This includes training on the technology itself, relevant manufacturing processes, quality control procedures, and regulatory requirements. Examples include hands-on training on new equipment, theoretical lectures on the underlying science of the technology, and workshops on regulatory compliance.
- Knowledge Transfer: Facilitate knowledge transfer from the technology provider to the recipient organization. This may involve mentorship programs, technical assistance, and ongoing support. This can be achieved through peer-to-peer mentorship, knowledge sharing sessions, and access to technical experts.
- Continuous Learning: Promote a culture of continuous learning and development. This can involve providing opportunities for ongoing training, professional development, and knowledge sharing.
Effective Project Management
Effective project management is crucial for ensuring the timely and efficient completion of the technology transfer process.
- Project Plan: Develop a detailed project plan that Artikels the scope, timeline, budget, and resources required for the transfer. This plan should be reviewed and updated regularly to ensure it remains aligned with the project’s objectives.
- Resource Allocation: Allocate sufficient resources, including personnel, equipment, and funding, to support the technology transfer process. This includes assigning clear roles and responsibilities to ensure accountability and efficient execution.
- Risk Management: Proactively identify and manage potential risks throughout the project lifecycle. This involves developing and implementing mitigation strategies to minimize the impact of any unforeseen challenges.
Ongoing Monitoring and Evaluation
Continuous monitoring and evaluation are essential to ensure the success of the technology transfer process.
- Performance Indicators: Establish key performance indicators (KPIs) to track the progress and effectiveness of the transfer. This may include metrics such as production output, quality control data, and regulatory compliance.
- Regular Reviews: Conduct regular reviews to assess the effectiveness of the transfer process and identify any areas for improvement. This can be achieved through data analysis, feedback mechanisms, and stakeholder meetings.
- Continuous Improvement: Use the findings from monitoring and evaluation to continuously improve the technology transfer process. This may involve making adjustments to training programs, communication protocols, or project management strategies.
Impact of Technology Transfer on Pharmaceutical Innovation
Technology transfer plays a pivotal role in driving pharmaceutical innovation, facilitating the translation of scientific discoveries into life-saving drugs and therapies. It bridges the gap between research and development, enabling the rapid advancement of new treatments and improving patient outcomes.
Impact on Drug Development
Technology transfer significantly impacts the development of new drugs and therapies. It allows for the transfer of expertise, knowledge, and technologies from research institutions to pharmaceutical companies, accelerating the process of drug discovery and development. By sharing knowledge and resources, technology transfer fosters collaboration and innovation, leading to faster development cycles and the introduction of new treatments to the market.
Examples of Successful Technology Transfer
Technology transfer has played a crucial role in the development of numerous groundbreaking drugs and therapies.
- The development of the first monoclonal antibody therapy, “Orthoclone OKT3”, for the treatment of acute organ rejection in transplant patients, was a direct result of technology transfer from the National Institutes of Health (NIH) to Ortho Pharmaceutical Corporation. This transfer of knowledge and technology led to a significant advancement in transplantation medicine, enabling the safe and effective use of organ transplantation for patients with end-stage organ failure.
- The development of the blockbuster drug “Lipitor” (atorvastatin), a cholesterol-lowering medication, involved technology transfer from the University of California, San Francisco (UCSF) to Pfizer. The transfer of UCSF’s research findings and intellectual property enabled Pfizer to develop and commercialize Lipitor, which became one of the best-selling drugs in history.
- The development of “Herceptin” (trastuzumab), a targeted therapy for HER2-positive breast cancer, was facilitated by technology transfer from the University of California, Los Angeles (UCLA) to Genentech. UCLA’s research on the HER2 protein led to the development of Herceptin, which revolutionized the treatment of breast cancer and significantly improved survival rates for patients with this aggressive form of the disease.
Addressing Unmet Medical Needs
Technology transfer plays a crucial role in addressing unmet medical needs by facilitating the development of treatments for rare diseases, neglected tropical diseases, and other conditions that have historically received limited attention from pharmaceutical companies.
- Technology transfer to developing countries can help address the global burden of diseases like malaria, tuberculosis, and HIV/AIDS. The transfer of technologies and expertise can empower local researchers and manufacturers to develop and produce affordable and accessible treatments for these diseases.
- Technology transfer can also facilitate the development of orphan drugs, which are medications for rare diseases that often lack sufficient market incentives for pharmaceutical companies to invest in their development. By transferring expertise and resources, technology transfer can help overcome these barriers and bring life-saving treatments to patients with rare diseases.
Wrap-Up
Technology transfer in the pharmaceutical industry is a dynamic and evolving field, shaped by emerging trends such as digitalization, open innovation, and artificial intelligence. These advancements offer exciting possibilities for accelerating drug development and improving patient outcomes. As the industry navigates these changes, a collaborative approach that fosters transparency, communication, and knowledge sharing will be essential for ensuring the successful transfer of innovative technologies to benefit patients worldwide.
Technology transfer in the pharmaceutical industry is crucial for bringing new treatments to market. This process often involves collaboration with specialized companies like spring technologies inc , who can provide expertise in areas such as manufacturing, quality control, and regulatory compliance.
By leveraging the strengths of these external partners, pharmaceutical companies can streamline their technology transfer processes and accelerate the development and commercialization of life-saving medications.